|
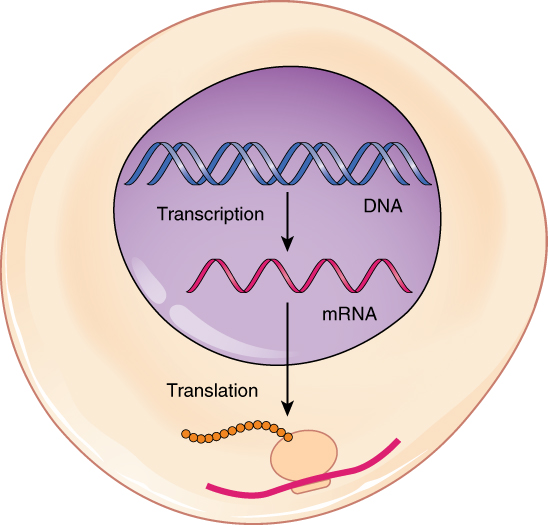
| Structure of DNA |
They pair to make a code, which can be replicated through semi-conservative replication during interphase, or be transcribed by helicase and RNA polymerase to create mRNA used in protein synthesis. The mRNA is translated into amino acids in a ribosome, and the resulting protein is sent to use in the cell or body. However, mutations can cause significant changes in DNA that lead to radically different proteins being produce, and this can interfere with processes. Additionally, gene expression is regulated by transcription factors and repressors, keeping certain genes from being read by RNA polymerase. This controls the production of certain proteins, such as lactose, from being produced unless they are needed. In general, much of this was a review for me, but there were also times where I took longer to understand new concepts that were presented, such as how operators work in gene expression and regulation. On the other hand, the structure of DNA and protein synthesis were not too challenging to grasp, especially since I had encountered them before in science classes, though the practice was very helpful.
 |
| DNA extracted during the DNA extraction lab |
Throughout the unit, I found that I learn better when asking questions and clarifying more complex parts of the content. I discovered that for topics like gene regulation and expression, I tended to understand the material and process much better after the in-class discussion the next day. As a student, I would consider myself having improved, as I learned how to garner more information and skills from what is presented; in essence, I am not only smarter now than a month (or the duration of the unit) ago, but I have improved at improving. (Δ(Δk)?) I think the strategy of physically typing and creating a study guide is an effective one: through writing down the material, one is reviewing and remembering perhaps nuances that were forgotten over the course of a few months. Next semester, I will try to utilize this method to synthesize material better and continue to learn more. (and learn more about learning)